

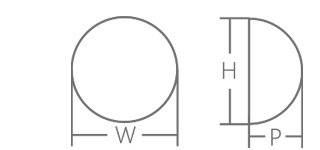

| REF | Width(W)±3mm | Height(H)±3mm | Profile(P)±5mm |
| DHSLP140 | 96mm | 87mm | 35mm |
| DHSLP160 | 100mm | 91mm | 36mm |
| DHSLP180 | 105mm | 96mm | 38mm |
| DHSLP200 | 108mm | 99mm | 39mm |
| DHSLP220 | 110mm | 101mm | 40mm |
| DHSLP240 | 115mm | 106mm | 42mm |
| DHSLP260 | 118mm | 109mm | 43mm |
| DHSLP280 | 120mm | 111mm | 44mm |
| DHSLP300 | 122mm | 113mm | 45mm |
| DHSLP330 | 125mm | 116mm | 46mm |
| DHSLP360 | 130mm | 121mm | 48mm |
| DHSLP390 | 135mm | 126mm | 50mm |
| DHSLP420 | 138mm | 129mm | 52mm |
| DHSLP450 | 142mm | 133mm | 53mm |
| DHSLP480 | 145mm | 136mm | 54mm |
| REF | Width(W)±3mm | Height(H)±3mm | Profile(P)±5mm |
| DHSMP180 | 98mm | 103mm | 36mm |
| DHSMP200 | 101mm | 106mm | 37mm |
| DHSMP220 | 104mm | 109mm | 38mm |
| DHSMP240 | 107mm | 112mm | 39mm |
| DHSMP260 | 110mm | 115mm | 41mm |
| DHSMP280 | 113mm | 118mm | 43mm |
| DHSMP300 | 116mm | 121mm | 45mm |
| DHSMP325 | 120mm | 125mm | 45mm |
| DHSMP350 | 125mm | 130mm | 46mm |
| DHSMP375 | 127mm | 132mm | 47mm |
| DHSMP400 | 130mm | 135mm | 48mm |
| DHSMP440 | 135mm | 140mm | 49mm |
| DHSMP480 | 140mm | 145mm | 50mm |